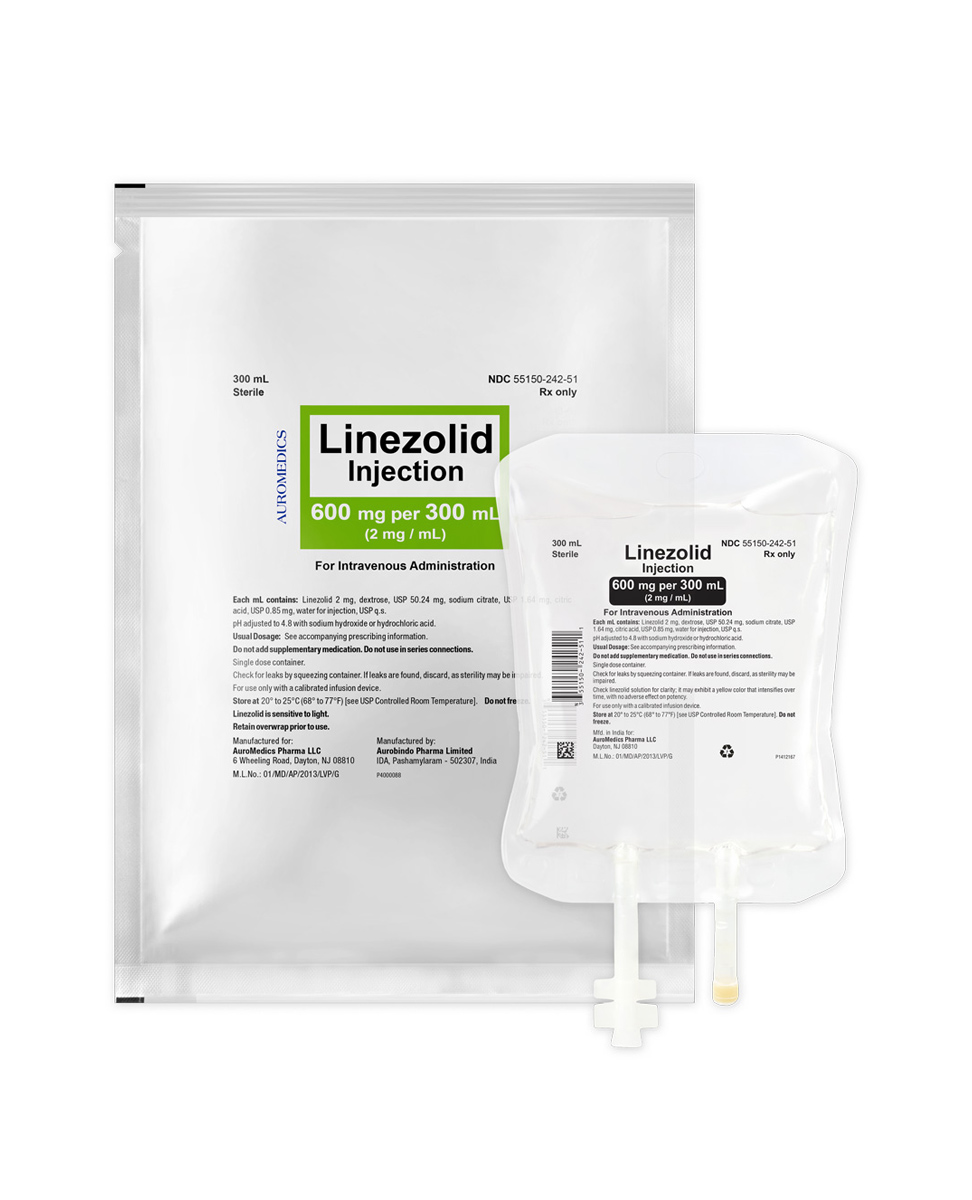
Linezolid Injection Premixed Bags
Overview
- Presentation(s):
-
600 mg/300 mL



- NDC(s):
-
55150-0242-51
- Reference Listed Drug:
- Zyvox®
- Therapeutic Class:
- Antibacterial
- Physical Form:
- Premixed Bags
Product Information
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-0242-51 | Premixed Bag | 600 mg/300 mL | 2 mg/mL | 300 mL | 300 mL | N/A |
Wholesaler Item Numbers
ABC
10169263Cardinal
5290879McKesson
3588423M&D
855346Ordering Information
Unit of Sale
10 bagsPack Size
10Case Quantity
1Allergens
Additional Information
Bioequivalency Rating
APBar Code Type
LinearControlled Substance Class
N/AStorage Requirements:
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]For Package Inserts, please click on the NDC#
Our products are available from authorized distributors.
For complete ordering information, please contact your local pharmaceutical distributor.