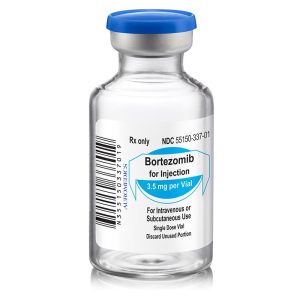
Bortezomib for Injection
Overview
- Presentation(s):
-
3.5 mg/vial



- NDC(s):
-
55150-337-01
- Reference Listed Drug:
- VELCADE®
- Therapeutic Class:
- Antineoplastic
- Physical Form:
- Powder
Product Information
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-337-01 | SD vial | 3.5 mg/vial | – | 10mL | – | – |
Wholesaler Item Numbers
ABC
10267384Cardinal
5783527McKesson
2611283M&D
218594Ordering Information
Unit of Sale
1Pack Size
1Case Quantity
48Allergens
Additional Information
Bioequivalency Rating
APBar Code Type
LinearControlled Substance Class
N/AStorage Requirements:
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Retain in original package to protect from light.For Package Inserts, please click on the NDC#
Our products are available from authorized distributors.
For complete ordering information, please contact your local pharmaceutical distributor.